Alliance for Artificial Intelligence in Healthcare Presents at BIO’s Clinical Trial Diversity Roundtable
BIO Roundtable works toward greater clinical trial diversity


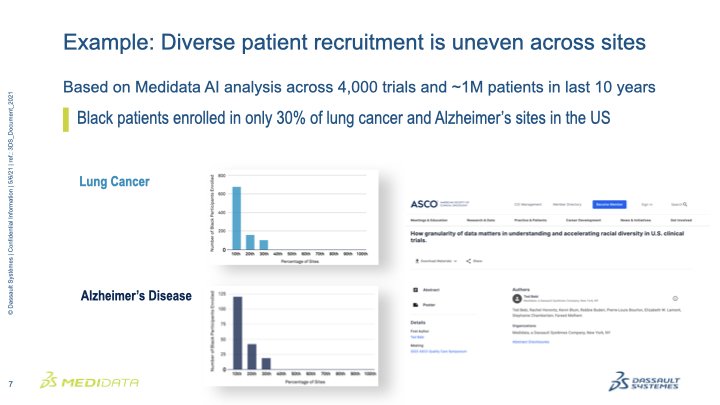

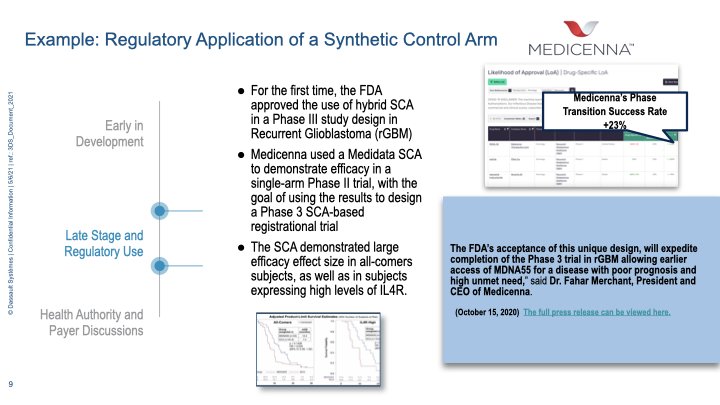
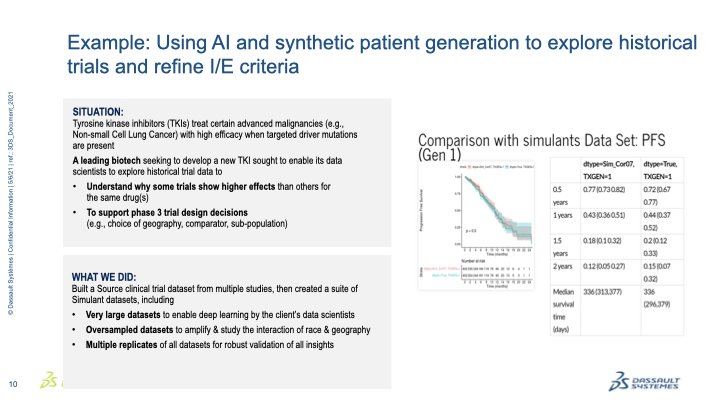

February 1, 2023 – Yesterday, the Biotechnology Innovation Organization’s (BIO) Clinical Trial Diversity Roundtable brought together leaders from government, industry, and patient groups to begin implementing the clinical trial diversity provisions of the 2022 omnibus spending package.
“At the outset, we intended to have an honest, solutions-oriented discussion that not only highlighted the barriers in recruiting and retaining more diverse patients and workforces, but more so doing something so that our interest in diversifying clinical trials and workforces is not a performative notion, but a momentous movement,” said Leslie Harden, Pharm.D., Director of BIO’s Science and Regulatory Team.
“This must become business as usual,” she said in her inspiring closing remarks.
What is the Omnibus about clinical trials?
Sections 3601-3604 cover clinical trial diversity. The provisions include requirements for sponsors to develop and implement action plans and for the U.S. Food and Drug Administration (FDA) to consult with sponsors and stakeholders to convene public workshops on increasing enrollment of historically underrepresented populations in trials.
The goal of yesterday’s roundtable was to develop action plans for using data to improve trial diversity and ensuring diversity among the people running the trials.
Speaking during the roundtable, Aisling McDonough, Chief of Staff for U.S. Rep. Anna Eshoo (D-CA), said that BIO has been one of the few organizations providing real assistance in drafting the law’s language regarding clinical trial diversity.
“BIO is working hard to continue to advance our diversity agenda,” said BIO Chief Scientific Officer Dr. Cartier Esham, explaining that yesterday’s roundtable was the second in a series of BIO summits on clinical trial diversity.
Why is Clinical Trial Diversity Important?
Despite bearing a disproportionate burden for some diseases, racial and ethnic minorities are commonly underrepresented in biomedical studies, Bio.News wrote recently.
“Clinical trials provide an important foundation of information for determining whether a medical product is safe and effective; hence, enrollment in clinical trials should reflect the variety of the community that may eventually utilize the treatment,” said FDA.
“Black African Americans make up 43% of the lupus population but only 14% of all clinical trial participants” for lupus therapies, said Joy Buie of the Lupus Foundation of America.
Throughout the day, participants discussed scientific, moral, and equity reasons for improving diversity, with diversity proving time and again to be good for science and good for business.
“I want to remind you of the reality and impact of inaction: there are people that look like me, live like me, and need care like you and me who are right now being omitted by outdated scientific practices,” said BIO’s Harden to conclude the day.
“Certainly, it is morally the right thing to do to be inclusive of all people whenever possible, but as [Travere Therapeutics CEO] Eric Dube stated at the outset today, it is clearly equally good for science and, therefore, good for business,” continued Harden. “And as [National Minority Quality Forum CEO] Gary Puckerein aptly reminded us, going forward, if your clinical trial isn’t diverse, your competitor’s clinical trial will be, so set yourself up for success in business, too!”
ABOUT AAIH
The Alliance for Artificial Intelligence in Healthcare (AAIH) is an international advocacy organization dedicated to the responsible adoption and application of AI/ML in healthcare. Our members represent all healthcare sectors, including drug R&D, digital health, medical devices, healthcare delivery, data science & technology providers with a shared goal of improving the state of healthcare globally through AI-enabled solutions. For more information, please visit www.theaaih.org
